
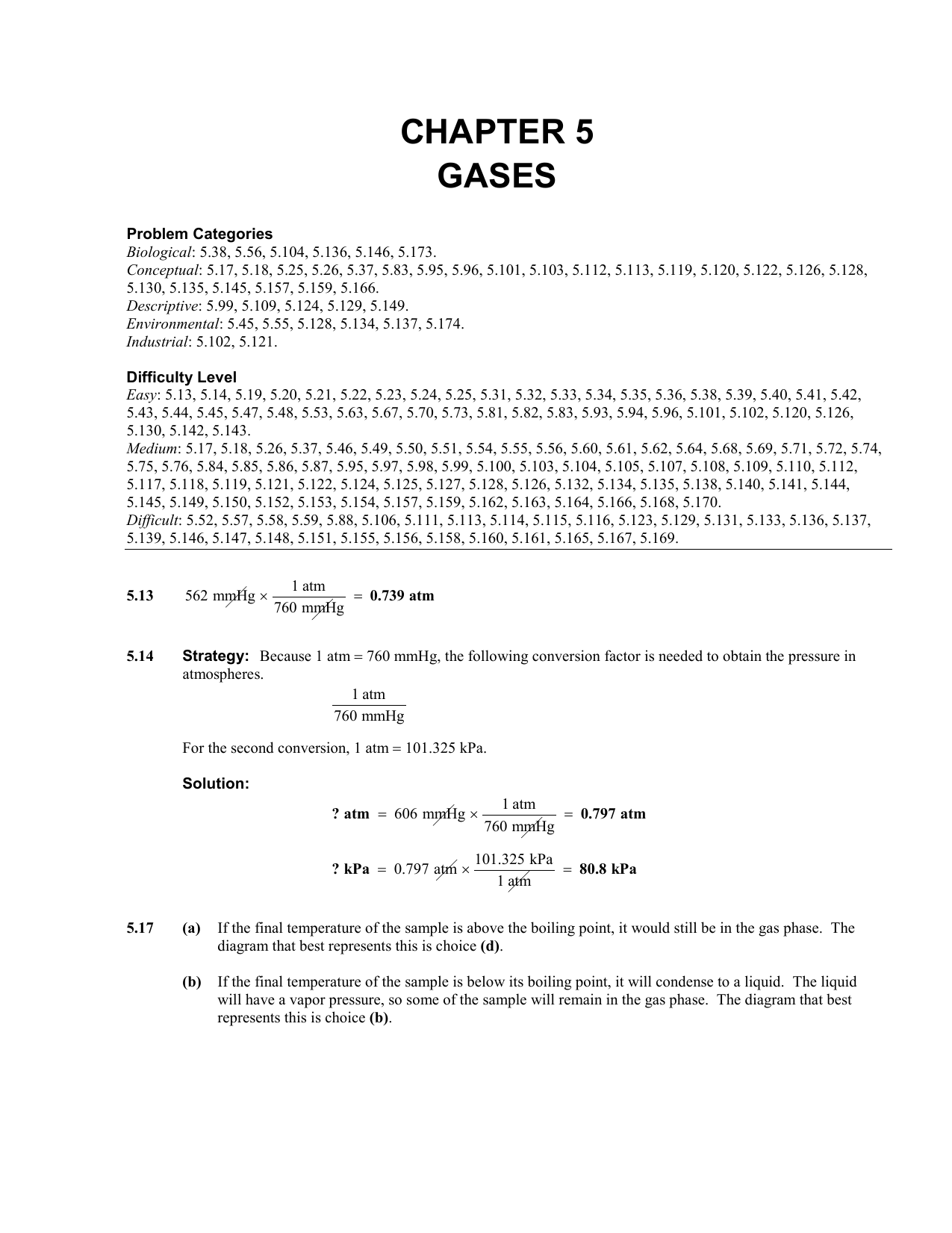
The atmospheric pressure at sea level is 14.7 psi. Atmospheric pressure is also often stated as pounds/square inch (psi). Standard atmospheric pressure is called 1 atm of pressure and is equal to 760 mmHg and 101.3 kPa. Determine the volume of nitrogen at 0 C and 760mmHg. What is the total pressure (in atm) when the valve is opened 18. 10.1 cm 746 mmHg 296 K3 760 mmHg 273 K ii i ff f VP T VP T (23 273) K 296 K (0 273) K 273 K i f T T + + Practice: According to the Dumas method of determining N in an organic compound, 39.8 mg of caffeine gives 10.1 cm3of nitrogen gas at 23 C and 746 mmHg. I get something like 420 mm (but the problem. X propane (g/44)/(g/44)+(g/58) Solve for X and substitute into the above. molar mass C3H8 about 44 molar mass C4H10 about 58. So all we need to do is to determine X propane and plug into the above. Q: How many Hectopascals in 746 Millimeters of Mercury The answer is 994.58. P total 746/760 atm (the problem asks for atm). More information from the unit converter. The right hand cylinder contains 3.0 L of He at 0.60 atm and the left hand cylinder contains 6.8 L of He at 2.0 atm. 746 Millimeters of Mercury (mmHg) 994.58 Hectopascals (hPa) 1 mmHg 1.333220 hPa. Assume two cylinders at 27 C are connected by a closed valve system. Another commonly used unit of pressure is the atmosphere (atm). (1 atm 760 mm Hg) Save rounding for the end.
A pascal is a very small amount of pressure, so the more useful unit for everyday gas pressures is the kilopascal (kPa).

The pascal (Pa) is the standard unit of pressure. After purification its volume was found to be 19.4 L. 1500 mmHg Page number 18 Chapter V : Gaseous State TareQ FoQha f 20192018 ELECTRICAL ENGINEERING COMMITTEE. If the volume remains constant, the pressure will change from 750 mmHg to : A. An equivalent unit to the mmHg is called the torr, in honor of the inventor of the barometer, Evangelista Torricelli. A gas evolved during the fermentation of alcohol was collected at 17C and 746 mm Hg. 61.0 L 14- The temperature of a gas in a sealed container changes from 40 ☌ to 80 ☌. One unit of gas pressure is the millimeter of mercury (mmHg). Tire pressure is best measured when the tire is cold since driving the car for a while will heat up the air in the tire and increase the pressure.Ī barometer measures gas pressure by the height of the column of mercury. The pressure on the tire is the maximum pressure for that tire, not the recommended one.
746 mmhg to atm manual#
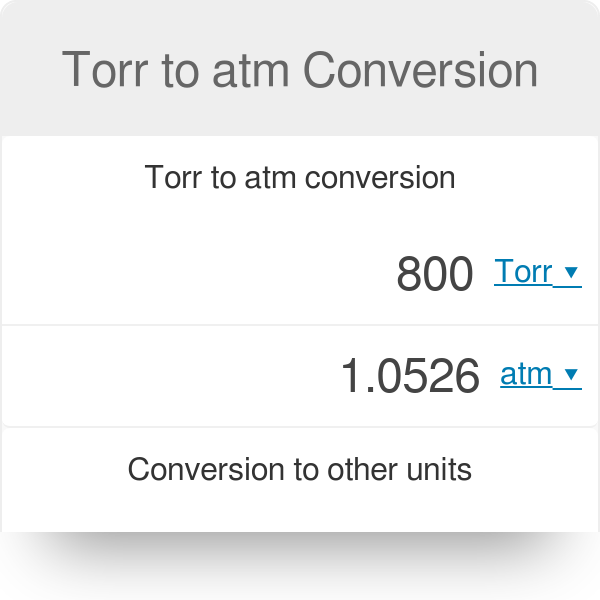
The recommended pressure for that model of car (usually somewhere between 32-35 psi) is usually listed in the owner’s manual or stamped somewhere inside the door. The car gets better gas mileage and the tires don’t wear out as fast. The ride is smoother and safer than with lowered pressure.
There are several benefits to maintaining the proper air pressure in a car tire.


 0 kommentar(er)
0 kommentar(er)
